IntraSight interventional platform
Best-in-class interventional tools built for today’s lab, ready for tomorrow’s innovations
Introducing Philips IntraSight interventional applications platform where imaging, physiology, co-registration* and software come together to facilitate optimal patient care. IntraSight offers a comprehensive suite of clinically proven modalities, such as, iFR/FFR, IVUS and co-registration* to simplify complex interventions, speed routine procedures and provide improved patient care.
Features

Advanced protection against cybersecurity threats
With the most robust cybersecurity, which runs on Microsoft Windows 10, providing you with the latest data encryption and protection against cybersecurity threats.

Built on a smart, applications-based platform
IntraSight can scale to meet the evolving needs of your lab when new applications or modalities become available, without the need to purchase new hardware. Only Philips Interventional Applications Platform, IntraSight, offers best-in-class imaging and physiology tools with iFR, iFR Co-registration*, FFR, IVUS, IVUS Co-registration*, and Angio+*. With its modular architecture, IntraSight stays on top of latest advances and important security updates.
Minimize learning curves and increase workflow confidence
Increase case efficiency, save time and reduce errors with seamless data

Optimizing lab performance with tableside touchscreen control, systems integration, data management and remote service diagnostics

Easy-to-use, small footprint, digital IVUS imaging and physiology system
IntraSight Mobile is designed for peripheral vascular and coronary procedures, operable directly from the sterile field. This is available for the hospital, office-based lab, and ambulatory surgery center environment.
IntraSight interventional platform technology is available on
-
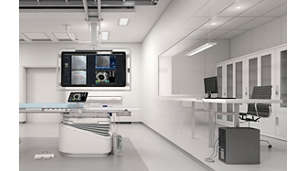
IntraSight
The IntraSight Interventional Applications Workspace is where imaging, physiology, co-registration* and software all come together to help identify coronary and peripheral artery disease, and allow for more optimized treatment plans. IntraSight is built on a new foundational platform designed to meet the evolving needs of your lab today and tomorrow.
IGTDINTRSGHT -
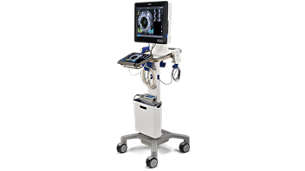
IntraSight Mobile
IntraSight Mobile is an easy-to-use, small footprint, digital IVUS imaging and physiology system, designed for peripheral vascular and coronary procedures, operable directly from the sterile field. This is available for the hospital, office-based lab, and ambulatory surgery center environment.
IGTDINTRSGHTMBL

Class IA recommendation
Includes iFR physiology the global gold standard among resting indices with a Class IA recommendation in the ACC/AHA/SCAI and ESC Guidelines.2,3
Documentation
- Resources
-
- Video assets
-
Footnotes
* Co-registration tools available within IntraSight 7 configuration via SyncVision.
* Philips Mobile Interventional Applications Platform – IntraSight Mobile – is CE marked for sale in Europe and has received FDA 510(k) clearance for sale in the U.S.
[1] Co-registration tools available within IntraSight 7 configuration via SyncVision.
[2] Lawton J. et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization. JACC. 2022;79(2):e21-e129.
[3] 2018 ESC/EACTS Guidelines on myocardial revascularization: The task force on myocardial revascularization of the European society of cardiology (ESC) and European association for cardio-thoracic surgery (EACTS). Eur Heart J. 2018;00:1-96.
Disclaimer
Always read the label and follow the directions for use.
Philips medical devices should only be used by physicians and teams trained in interventional techniques, including training in the use of this device.
Products subject to country availability. Please contact your local sales representative.
©2024 Koniklijke Philips N.V. All rights reserved. Trademarks are the property of Koninklijke Philips N.V. or their respective owners. Philips reserves the right to change product specifications without prior notification.